Medical device
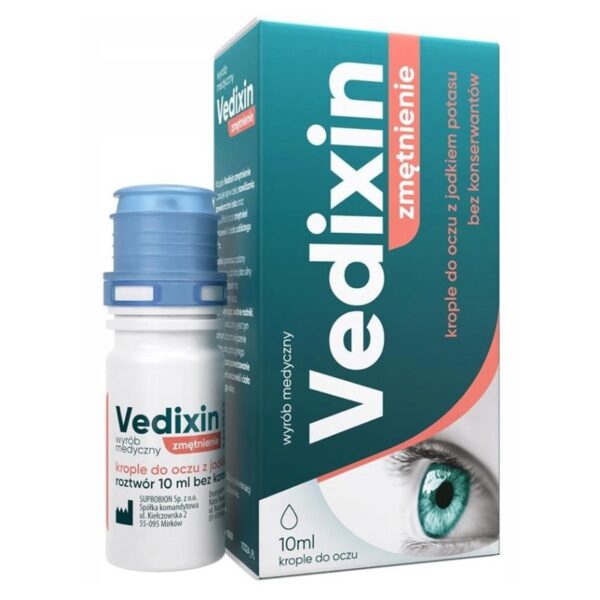
Vedixin Turbidity eye drops with potassium iodide, 10 ml
Vedixin drops turbidity are eye drops with potassium iodide. Sterile eye drops, preservative-free.
Destiny:
- Potassium iodide eye drops.
- Moisturizing the surface of the eye, preventing clouding of the lens and vitreous body.
- Can be used by contact lens wearers.
Properties:
- Vedixin opacities work in two directions: moisturizing the eye surface, preventing opacification of the lens and vitreous body of the eye.
- Potassium iodide 5mg/ml has a strong antioxidant effect on the surface of the eye, which reduces oxidative stress. It helps to limit the process of clouding of the lens and vitreous body.
- Sodium hyaluronate 1mg/ml with moisturizing properties combines with tear fluid during blinking, retains water and has mucoadhesive properties, thanks to which it delays the evaporation of water from the surface of the eye and stabilizes the tear fluid.
- Vedixin opacification drops provide protection and prevention of the eye with the problem of opacification of the lens and vitreous body.
How to use:
Apply 1 drop to each conjunctival sac 3-4 times a day, and in case of severe symptoms you can apply the drops more often.
- Can be used by people wearing contact lenses.
- The drops can be used daily because they do not contain preservatives.
- It is recommended to use the drops for 3 months after first opening the container, no longer than one month continuously and no longer than 3 months when used intermittently.
- When using other ophthalmic preparations, it is recommended to use Vedixin drops last.
Composition:
Sodium hyaluronate 1mg/ml, potassium iodide 5mg/ml, boric acid, sodium tetraborate, sodium chloride, highly purified water.
10 ml solution without preservatives.
Important:
- Do not use in case of hypersensitivity to any of the ingredients of the preparation.
- If an allergic reaction occurs, discontinue use of the preparation.
- Keep out of the reach of children.
- Store in a dry place at a temperature between 5°C and 35°C.
- Do not use after the expiration date printed on the packaging.
- The preparation is intended for external use only.
- Never touch the dropper tip to your eye or other surfaces as this may contaminate the solution.
- A reusable medical device should only be used by one person.
Attention:
- This is a medical device. Use it according to the instructions for use or label.
- Medical device – CE 0051 certified
- It has an EU declaration of conformity .
- Includes user manual in Polish.
- Contains a label and markings in Polish.
- Storage and transport of medical devices are in accordance with the conditions specified by the manufacturer.
Producer:
SUPROBION Sp. z o. o., Sp. k., ul. Kiełczowska 2, 55-095 Mirków.
Distributor:
Natur Produkt Zdrovit Sp. z o. o., ul. Nocznickiego 31, 01-918 Warsaw.


































Reviews
Clear filtersThere are no reviews yet.